How to Balance:
Mg(OH)2 + HNO3 → Mg(NO3)2 + H2O
Word equation: Magnesium hydroxide + Nitric acid → Magnesium nitrate + Water
Type of Chemical Reaction: For this reaction we have a neutralization reaction.
Balancing Strategies: Here we have a neutralization reaction. The magnesium hydroxide and the nitric acid combined and form a salt and water. The salt is the magnesium nitrate. You could also call this a double displacement reaction.
Be careful when you count the number of hydrogen atoms. You have two in the magnesium hydroxide and one for the nitric acid.
Essential Resources
For a complete explanation, watch:
When balancing chemical equations our goal is to have the same number of each type of atom on both sides of the equation.
Only change the coefficients (these are the numbers in front substances).
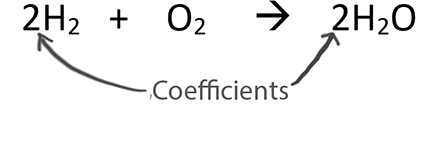
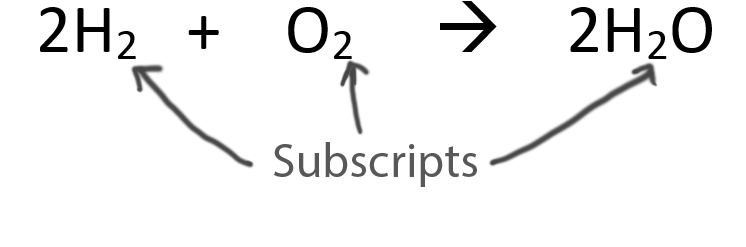
Video:
Subscribe