How to Balance Chemical Equations
A big reason we balance equations is to find the ratios between different substances in the chemical reaction. The coefficients, the big numbers in front of compounds, tell us the ratios. These are often thought of as mole ratios.
Practice Balancing
Key Information
When balancing chemical equations our goal is to have the same number of each type of atom on both sides of the equation.
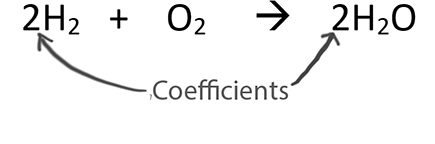
You can only change the coefficients when balancing!
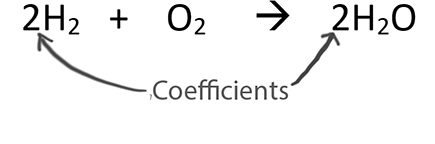
Coefficients in balanced equations provide ratios between substances. These can be thought of as mole ratios.