How to Balance:
H2 + O2 → H2O
Word equation: Hydrogen gas + Oxygen gas → water
Type of Chemical Reaction: For this reaction we have a Combination reaction.
Balancing Strategies: For this reaction it is helpful to start by changing the coefficient in front of H2O and so that you have an even number of oxygen atoms. After you do that you can go to the reactants side of the question (and the left side) and fairly easily, balance the rest of the equation.
This is a good reaction to make sure you understand since many introductory courses use this as an example. It's often used as a starting point in stoichiometry problems as well.
Essential Resources
For a complete explanation, watch:
When balancing chemical equations our goal is to have the same number of each type of atom on both sides of the equation.
Only change the coefficients (these are the numbers in front substances).
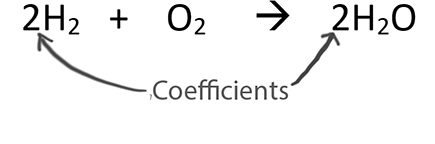
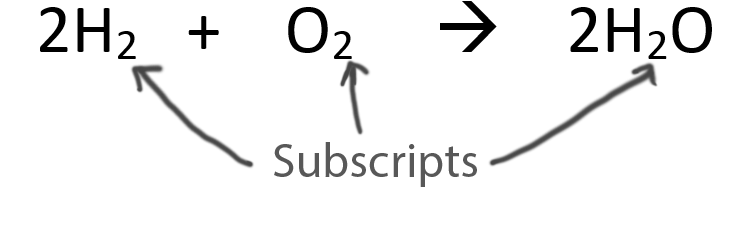
Video:
Subscribe