How to Balance:
CaO + HCl → CaCl2 + H2O
Word equation: Calcium oxide + Hydrochloric acid → Calcium chloride + Water
Type of Chemical Reaction: For this reaction we have a double replacement reaction.
Balancing Strategies: In this chemical equation we have a double replacement reaction.
Overall this is a straightforward equation to balance.
Essential Resources
For a complete explanation, watch:
When balancing chemical equations our goal is to have the same number of each type of atom on both sides of the equation.
Only change the coefficients (these are the numbers in front substances).
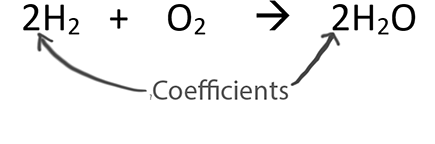
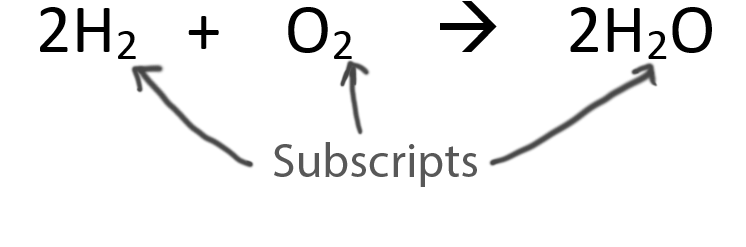
Video:
Subscribe